image credit: EUSKALANATO (Flickr)
What Cells Can be Made from Stem Cells?
Ever since the discovery of pluripotent stem cells (PSCs) more than 50 years ago and the isolation of the first-ever human embryonic stem cells (hESCs) about 20 years back, great advances have been made in the field of stem cell research. This has prompted the concept of directed differentiation, which now allows PSCs to be transformed into different tissue-specific cell types in vitro by carefully guiding and directing cells to adopt various cellular identities.
The transformation of the cells is usually done directly by either introducing specific transcription factors to PSCs, or indirectly by manipulating an amalgamation of signaling pathways that govern the expression of these transcription factors (by way of small molecules or signaling proteins). Regardless of the technique, the outcome is to faithfully recapitulate the complexities of in vivo development to drive PSCs to develop and differentiate into different cell fates.
Because PSCs are able to self-renew, they can expand and proliferate while maintaining pluripotency. This allows the ability to continuously differentiate and produce large quantities of different cell types, opening up doors to cellular transplantation therapies for the treatment of chronic diseases.
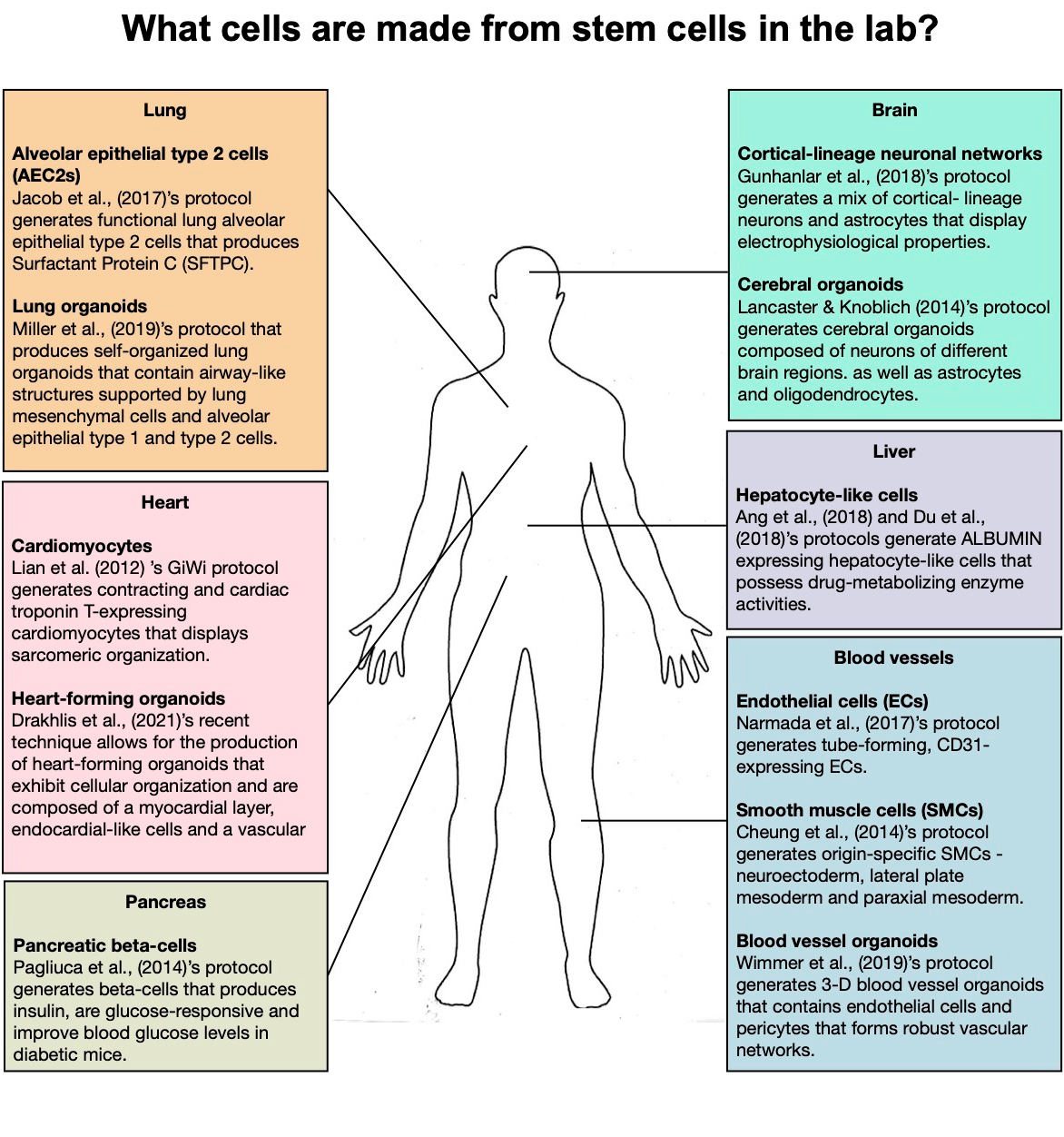
What cells can be made from stem cells in the lab? The last decade has seen an explosion of research that focuses on formulating strategies to direct differentiation of PSCs to all kinds of tissue cell types – from neurons to pancreatic beta cells, and even liver cells. One of the most common methodologies used is to perform step-wise differentiation where PSCs are directed to follow a certain developmental trajectory one stage at a time.
Brain
To create functional and mature neurons from human PSCs (hPSCs), hPSCs must first be differentiated into the neuroectoderm lineage and neural progenitor cells (NPCs). This can be achieved by an efficient and simple dual SMAD inhibition method described by Chambers et al. in 2009 [1]. NPCs can then be directed to develop into specific neurons through different combinations of signals [2,3]. At the end of the process, the differentiated neurons will express neuronal markers such as TUJ1 and MAP2 and some PSC-derived neurons can even produce electrophysiological responses [4]. Gunhanlar et al. (2018) described a method to differentiate induced human PSCs (iPSCs) into cortical-lineage neuronal network composed of both neurons and astrocytes that originate from a common forebrain neural progenitor [5]. The differentiated neurons display mature electrophysiological properties just like neurons in the body, such as resting membrane potential and action potential. Researchers have also devised a method to generate ‘mini-brains’ from PSCs. A protocol described by Lancaster & Knoblich (2014) differentiates hPSCs in a step-wise fashion into cerebral organoids that exhibit spatial organization (like the brain) and stains for brain regions such as forebrain, choroid plexus, and hippocampus [6].
Lungs
Jacob et al.’s (2017) protocol allows for the generation of functional lung alveolar epithelial type 2 (AT2) cells that produce Surfactant Protein C (STPC), a hydrophobic surfactant protein that is produced specifically by AT2 cells [7]. In 3D cultures, these cells are even able to form monolayered epithelial ‘alveolospheres’. Miller et al. (2019) published a method on generating lung organoids from hPSCs by first patterning hPSCs to ventral-foregut spheroids and then to human lung organoids [8]. At the end of the differentiation process, the lung organoids contain structures resembling bronchi surrounded by mesenchymal cells and both AT1 and AT2 cells.
Heart
One of the popular protocols to generate cardiomyocytes from hPSCs is known as the ‘GiWi’ protocol because it involves the temporal modulation of the Wnt signaling pathway by first, inhibiting glycogen synthase kinase 3 “(GSK-3), followed by WNT. This simple protocol drives cardiac differentiation and is able to produce up to 98% pure functional cardiomyocytes that express cardiac troponin-T (an essential protein for cardiomyocytes to contract) and display sarcomeric organization.
Infographic Credit: Nicole Pek
Liver
There are numerous protocols available today to differentiate hPSCs into hepatocytes. Ang et al. (2018) and Du et al. (2018) both describe similar strategies to obtain hepatocytes from hPSCs by first driving differentiation into the definitive endoderm lineage, then to hepatic progenitors, and finally to hepatocyte-like cells [9,10]. The differentiated cells express key marker Albumin, an essential protein made by the liver, as well as Carbamoyl-phosphate synthase (CPS1), and Alpha-1 antitrypsin (AAT). These cells were also shown to possess drug metabolism enzyme activities.
Pancreas
Pagliuca et al. (2014) reported on a 3D-based method to derive insulin-producing pancreatic beta-cells from hPSCs [11] . This highly-cited protocol also utilizes the step-wise differentiation strategy where hPSCs were patterned into the definitive endoderm lineage, followed by further differentiation into pancreatic progenitors which are subsequently matured into pancreatic beta-cells. These cells were shown to respond to glucose stimulation, resemble human islet beta-cells and reverse hyperglycemia in mice models of diabetes.
Blood Vessels
Blood vessels are composed of vascular endothelial cells (ECs) and vascular smooth muscle cells (SMCs). There are protocols describing methods to generate both vascular cell types from hPSCs. For example, Narmada et al. (2017) described a method to differentiate ECs from hPSCs by directing cells into the mesodermal lineage under hypoxic conditions [12]. After differentiation, CD31-expressing ECs were purified. These cells are able to form tube-like structures in vitro. While the method generates a more generalized type of ECs, Rosa et al. (2019) recently reported on a method to produce specific subtypes of ECs, namely arterial and venous ECs (from arteries and veins respectively, which are distinct from one another [13]. These differentiated cells display mechanical properties similar to blood vessels.
In 2014, Cheung et al. described a comprehensive approach to generate embryonic origin-specific SMCs from hPSCs in a step-wise fashion, under chemically-defined conditions [14]. hPSCs were first directed into three different lineages – neuroectoderm, lateral plate mesoderm, and paraxial mesoderm. Then, these early precursors were further differentiated into contractile SMCs, thus resulting in the production of lineage-specific SMC subtypes that have different identities and are functionally unique. This is important in the context of disease modeling or even transplantation therapies where the appropriate SMC subtype should be used i.e. for neuroectoderm-SMCs should be used to study/ treat stroke.
Lastly, recently, Wimmer et al (2019) described a method to produce self-organizing human blood vessel organoids from hPSCs cardiac trophonin-T [15]. The organoids contain ECs and pericytes that form well-defined capillary networks enveloped by basement membrane. Post-transplantation, these organoids were also able to engraft into the host animal and form stable, mature, and perfusable vascular networks that are composed of arteries and veins. The blood vessel organoids are able to mimic the structure and function of human blood vessels.
What’s Next
Now that the field of stem cell research has reached a significant milestone of establishing robust methods to produce different cells from PSCs in vitro, the next steps are to improve the efficiencies of these methods to increase the purity of differentiated cells. This is critical in ensuring that the PSC-derived cells will be safe and suitable sources of cells for transplantation therapies.
About the Author:
Nicole Pek is a
stem cell biologist and enthusiastic science communicator. She has worked on using human pluripotent stem cells
to study cellular development in multiple organ systems, to model complex human diseases, and screen for therapeutics that could treat the diseases. Outside of the lab, Nicole plays a pro-active role in communicating to the public through her science blog ‘Two Cells’ and her education podcast ‘The Diploid Duo’.
Suggested Content:
|
|
The Case for Investing in Regenerative Medicine in 2021
|
Scientists now Better Understand Viral Mutations
|
|

|
Preventing the Immune System from Rejecting Gene Therapy
|
Type 1 Diabetes, Beginning of the End – Close to a Cure (Video)
|
References
1. Chambers SM, Fasano CA, Papapetrou EP et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat
Biotechnol 2009;27:275–80.
2. Li Y, Liu M, Yan Y et al. Neural differentiation from pluripotent stem cells: The role of natural and synthetic extracellular matrix. World J Stem Cells 2014;6:11–23.
3. Hong YJ, Do JT. Neural Lineage Differentiation From Pluripotent Stem Cells to Mimic Human Brain Tissues. Front
Bioeng Biotechnol 2019;7, DOI: 10.3389/fbioe.2019.00400.
4. Zhang Y, Pak C, Han Y et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013;
78:785–98.
5. Gunhanlar N, Shpak G, van der Kroeg M et
al. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol
Psychiatry 2018;23:1336–44.
6. Lancaster MA, Knoblich JA. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat Protoc 2014;9:2329–40.
7. Jacob A, Morley M, Hawkins F et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 2017;21:472-488.e10.
8. Miller AJ, Dye BR, Ferrer-Torres D et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat
Protoc 2019;14:518–40.
9. Ang LT, Tan AKY, Autio MI et al. A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells. Cell
Rep 2018;22:2190–205.
10. Du C, Feng Y, Qiu D et al. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res Ther 2018;9:58.
11. Pagliuca FW, Millman JR, Gürtler M et
al. Generation of functional human pancreatic ? cells in vitro. Cell 2014;
159:428–39.
12. Narmada BC, Goh YT, Li H et al. Human Stem Cell?Derived Endothelial?Hepatic Platform for Efficacy Testing of Vascular?Protective Metabolites from Nutraceuticals. Stem Cells Transl Med 2017;
6:851–63.
13. Rosa S, Praça C, Pitrez PR et al. Functional characterization of iPSC-derived arterial- and venous-like endothelial cells. Sci Rep 2019;9:3826.
14. Cheung C, Bernardo AS, Pedersen RA et
al. Directed differentiation of embryonic origin-specific vascular smooth muscle subtypes from human pluripotent stem cells. Nat Protoc 2014;9:929–38.
15. Wimmer RA, Leopoldi A, Aichinger M et
al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019;565:505–10.
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |
Stay up to date. Follow us:
 |
 |
 |
 |
 |
 |