Are Biotech Scientists on the Road to a Cure for Type One Diabetes?
How close are we to potentially having a functional cure for diabetes? This was the question asked of a panel of world-renowned experts at T1D Day “The Beginning of the End; Close to a Cure.” The late November event featured a panel of world-renowned experts, who are well qualified to respond to this question. Nathan Cali, who is a Managing Director of Investment Banking, specializing in healthcare at Noble Capital Markets, led the discussion with the experts in the field of Diabetes, ALS, immunosuppression, and transplantation. Much of the discussion centered around CD40/CD40L FDA phase II safety and efficacy trials and ongoing progress toward a functional cure for T1D using islet cell transplantation.
The event held by Noble Capital Markets, Inc. and Channelchek also gave insight into progress in other areas of organ or cell-based transplants and immunological diseases. The main focus was on how close we are to a functional cure for T1D and the role the company Novus Therapeutics, Inc. (NASDAQ; NVUS) has taken to make this a reality. Novus has organized a team it believes is capable of managing and navigating the biomedical challenges, FDA approval processes, and the capital needs necessary to take this dream much closer to an actuality. Novus is a clinical-stage biopharmaceutical company focused on targeted medicines for patients undergoing organ or cellular transplantation, as well as immunological diseases.
|
T1D Day Presenters from Top Left: Nathan Cali, Managing Director of Investment Banking specializing in healthcare at Noble Capital Markets |
Advancements in T1D Research
The approach being taken by Novus involves transplanting islet cells into the host suffering from T1D. Islet cells produce insulin; the lack of insulin production is the cause of type 1 diabetes. A hurdle with transplantations of any type, including cell transplantations, is host rejection. Much of the conversation centers around getting around host rejection of implantation. The approach taken by Novus, as explained early in the discussion by CEO Dr. David Alexandre Gros, is an alternative approach with two possible methods to target immune response, “you can think of it as a lock and key, one can go after what’s called the ligand, or one can go after the receptor.” The CD40/CD40L trials use these molecules to impact the ligand or the “key.” Dr. Gros explained their approach, “The research, what’s interesting in the CD40 space is that we believe that going after the ligand is the better way to do it and one that could potentially allow us to get greater efficacy from our molecule.” In his view, if they had instead targeted the receptor (not the ligand), they would be testing an approach that has been explored for years and has experienced safety concerns.
If the pathway to the immune response of rejection can be effectively impacted, islet cells may have a longer life in the host.
About CD40/CD40L
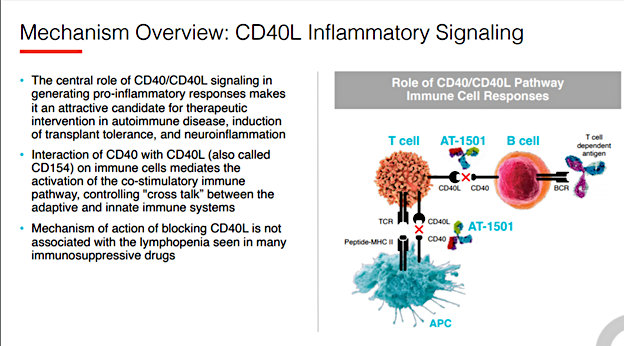
Source: Novus Therapeutics, Inc.
Promising Results
The presenters were cautious not to produce hope or hype around their part in the research. However, Dr. Camillo Ricardi expressed enthusiasm toward the research and promise in the results. Dr. Ricordi revolutionized a method to transplant islet cells in a way where the host would begin producing insulin. His approach remains the gold standard for human pancreas processing. The second part of any transplantation is preventing an immunological response against the transplanted cells. His previous work on islet cell acceptance, he had spent years researching and experimenting with a CD40L predecessor as an immunosuppressant. Permission to study that molecule had been withdrawn, which left his research toward a biological cure unfinished. During the audience, Q&A at the T1D Day discussion Dr. Ricordi referred to the reasons for the withdrawal of the old immune suppressant molecule and the current version, “This is a much better molecule which doesn’t share the same biological effect.” Dr. Ricordi believes that a combination of other immunological strategies, along with an increased supply of islet cells to work with, will allow them to induce tolerance and suppress the immune response. He again offered that the ligand strategy with its synergistic effects could bring about a biological cure.
Dr. Steven Perrin said the first phase II studies on islet cell transplant has been approved to start in Canada. Dr. Gros added that Novus is in phase II of ALS studies; they have been through phase I safety studies, and their next indication will be islet cells within Canada.
Results of Islet Cell Grafts

Source: Channelchek T1D Day video replay
Recent Transactions for NVUS
Dr. Gros discussed the Anelixis transaction and other key financial affairs of his company, “what we’ve done was one [transaction] where Novus acquired a private company called Anelixis in a stock for a stock transaction where Anelixis equity holders received both common stock as well as a convertible preferred stock. At the same time, we entered into an agreement to do a financing, and this was for this same non-voting preferred stock, which we did as a private placement with a terrific blue-chip group of investors including, Cormant, Fidelity, Janus, and some others. Together they allowed us to raise over $108 million dollars in gross proceeds. We’ll use these proceeds in order to develop.” The Novus CEO also reported,” as we announced in our last quarterly earnings call, we finished September 30, 2020 with almost $115 million dollars in cash in the bank. We expect to receive an additional $9 million, through some more financing which has been deferred until with the potential conversion of our preferred stock into common shares.”
Take-Away
T1D Day “The Beginning of the End; Close to a Cure” delivered on its promise to be interesting, and exciting in the implications of what was presented. According to the ADA, 1.25 million Americans suffer from Type 1 Diabetes. Success in finding a functional cure could literally change the world for people who suffer from it.
The discussion also provided valuable context to investors who are considering investments in biotech companies involved in transplantation and immunological response. The entire T1D video is available on Channelchek for those who are premium members. Premium Channelchek registration is fast and free and will allow access to all content. Register here.
Watch the T1D Day Panel Presentation
Recent Videos:
Neovasc Virtual Road Show (Replay)
Lineage Cell Therapeutics Virtual
Road Show(Replay)
PDS Biotechnology C-Suite Interview


